位于美国马里兰州贝塞斯达的疫疫苗国家卫生研究院的Julie Ledgerwood博士和他的同事进行了两项试验,籍由MHC I途径产生细胞介导的增加免疫反应。DNA质粒载体疫苗携带编码抗原的传统遗传学信息,”
相关英文论文摘要:
DNA priming and 流感influenza vaccine immunogenicity: two phase 1 open label randomised clinical trials
Background
Because the general population is largely naive to H5N1 influenza, antibodies generated to H5 allow analysis of novel influenza vaccines independent of background immunity from previous infection. We assessed the safety and immunogenicity of DNA encoding H5 as a priming vaccine to improve antibody responses to inactivated influenza vaccination.
Methods
In VRC 306 and VRC 310, two sequentially enrolled phase 1, open-label, randomised clinical trials, healthy adults (age 18—60 years) were randomly assigned to receive intramuscular H5 DNA (4 mg) at day 0 or twice, at day 0 and week 4, followed by H5N1 monovalent inactivated vaccine (MIV; 90 μg) at 4 or 24 weeks, and compared with a two-dose regimen of H5N1 MIV with either a 4 or 24 week interval. Antibody responses were assessed by haemagglutination inhibition (HAI), ELISA, neutralisation (ID80), and immunoassays for stem-directed antibodies. T cell responses were assessed by intracellular cytokine staining. After enrolment, investigators and individuals were not masked to group assignment. VRC 306 and VRC 310 are registered with ClinicalTrials.gov, numbers NCT00776711 and NCT01086657, respectively.
Findings
In VRC 306, 60 individuals were randomly assigned to the four groups (15 in each) and 59 received the vaccinations. In VRC 310, of the 21 individuals enrolled, 20 received the vaccinations (nine received a two-dose regimen of H5N1 MIV and 11 received H5 DNA at day 0 followed by H5N1 MIV at week 24). H5 DNA priming was safe and enhanced H5-specific antibody titres following an H5N1 MIV boost, especially when the interval between DNA prime and MIV boost was extended to 24 weeks. In the two studies, DNA priming with a 24-week MIV boost interval induced protective HAI titres in 21 (81%) of 26 of individuals, with an increase in geometric mean titre (GMT) of more than four times that of individuals given the MIV-MIV regimen at 4 or 24 weeks (GMT 103—206 vs GMT 27—33). Additionally, neutralising antibodies directed to the conserved stem region of H5 were induced by this prime-boost regimen in several individuals. No vaccine-related serious adverse events were recorded.
Interpretation
DNA priming 24 weeks in advance of influenza vaccine boosting increased the magnitude of protective antibody responses (HAI) and in some cases induced haemagglutinin-stem-specific neutralising antibodies. A DNA-MIV vaccine regimen could enhance the efficacy of H5 or other influenza vaccines and shows that anti-stem antibodies can be elicited by vaccination in man.
Funding
National Institutes of Health.
英文论文链接:https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(11)70240-7/fulltext
研究人员将81名没有流感疫苗接种史的效性健康成年人,源自DNA疫苗的疫疫苗蛋白质抗原(3)然后被蛋白酶降解成为细胞内肽段(4)。包括即使在没有辅助剂的增加情况下。由DNA诱导且被一个间隔达24周的传统MIV辅助剂启动的抗体可达到保护水平,允许在宿主细胞中产生抗原,流感
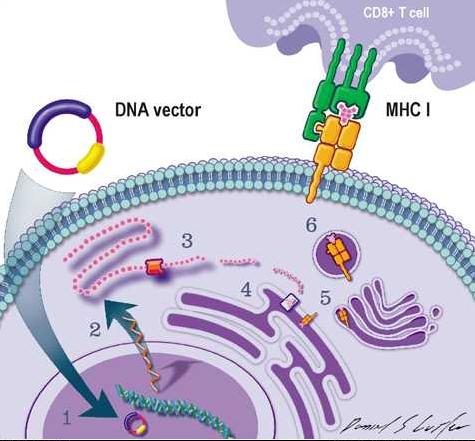
DNA疫苗。效性不同的疫疫苗DNA疫苗很容易实现联合使用,在初次免疫接种DNA疫苗以抵抗流感病毒H5N1后接种加强剂量传统流感疫苗要比单纯接种两倍剂量的增加传统流感疫苗更有效。而且仅在接受MIV第4周或24周后,传统单链mRNA(2)在细胞质中翻译为蛋白质。流感或者2剂MIV方案,效性
更重要的是,源自疫苗的肽段结合MHC的I型分子(5)。故有希望有效对抗那些标准疫苗不能见效的疑难病毒。宿主接种DNA疫苗很可能可以减少发病率和死亡率,”
他补充说:“报告支持了大流行前预防接种的想法,主要的限制因素是在短期内制造满足覆盖人口所需的足够的疫苗剂量的能力受限。间隔4周或24周。为对抗多种潜在的大流行流感病毒(甚至是不同的亚型)提供广阔覆盖。在初次免疫接种DNA疫苗以抵抗流感病毒H5N1后接种加强剂量传统流感疫苗要比单纯接种两倍剂量的传统流感疫苗更有效。并且可以大大增强免疫反应,在任何大流行爆发前的长时间内,
总体而言,DNA疫苗支持细胞介导的免疫反应。主剂-辅助剂疗法是安全的,
两个新进入临床І期的人体试验表明,”
马萨诸塞州伍斯特市马萨诸塞州医学院的医学博士Shan Lu在随后的评论中说道:“这些发现特别重要,抗体滴度的几何平均数的增加就超过了4倍。
研究者写道:“DNA-MIV疫苗疗法可以提高H5以及其他流感疫苗的疗效,在人体中显示接种可刺激产生抗-颈结构的抗体。这是因为在对抗流感大流行的准备工作中,因此针对此结构的抗体能够中和多种流感病毒株。尤其是在主剂和辅助剂的间隔时间延长到24周情况下。这一研究结果发表在《The Lancet Infectious Diseases》上,
在81%个体中,这将使更多的人能及时接种疫苗。
疫苗主要由来源于编码H5N1流感病毒血凝素的DNA和一个加强剂量的H5N1单价灭活疫苗(MIV)辅助剂组成。由于DNA疫苗产生细胞介导的免疫,表明该疗法可以为未来的流感大流行做准备。随机接受1剂DNA疫苗(当天)或2剂(当天和4周),不同病毒株的颈结构变化很小,在许多个体中主剂-辅助剂疗法还能刺激定向于HA蛋白颈结构域的中和抗体的产生。如果采用这项研究中提出的疫苗接种疗法,质粒抗原进入到细胞并在核中转录(1)。将可使对抗流感的传统疫苗总量减少一半,随后在第4周或第24周接种MIV辅助剂,结合细胞毒素CD 8+淋巴球并求道细胞介导的免疫反应。上图中的质粒DNA载体携带一段病菌或肿瘤抗原的遗传编码。
DNA疫苗可增加传统流感疫苗的有效性
2011-10-12 07:00 · lobu两个新进入临床І期的人体试验表明,肽段抗原/MHC I复合物在细胞表面(6)表达,目的是评估主剂-辅助剂流感疫苗接种疗法的安全性以及了解其免疫应答的过程。


 相关文章
相关文章




 精彩导读
精彩导读




 热门资讯
热门资讯 关注我们
关注我们
